News and Trends 25 Aug 2022
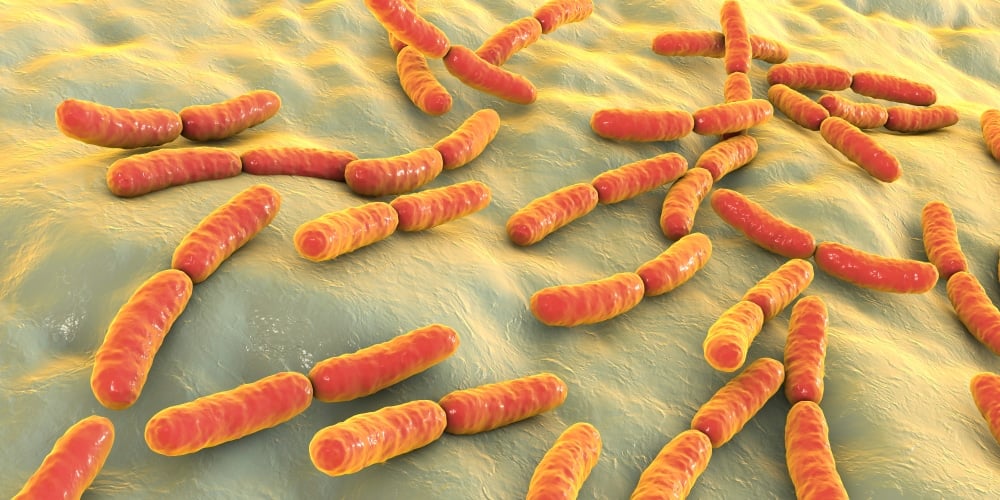
Ilya Pharma finalizes global patent coverage for modified lactic acid bacteria to treat wounds
Ilya Pharma, a Swedish clinical stage biopharma company focused on delivering local immunotherapies to patients, has announced the issue of four new patents. These are: one in India, and continuation patents in China, Australia and the U.S. This means the company’s method for using modified bacteria for treatment of both mucosal and cutaneous wounds in […]